Green Chemistry, a contribution to Sustainable Development
Sustainable development![]() ,
the keystone of technological progress in this new century, challenges chemical
sciences to play a primary role in converting old technologies into new clean
processes and in designing new products and new eco-compatible processes.
,
the keystone of technological progress in this new century, challenges chemical
sciences to play a primary role in converting old technologies into new clean
processes and in designing new products and new eco-compatible processes.
Green chemistry![]() ,
that is to say sustainable chemistry, is the new philosophy of chemistry,
whose aim is to correct present practices in order to prevent problems in the
future.
,
that is to say sustainable chemistry, is the new philosophy of chemistry,
whose aim is to correct present practices in order to prevent problems in the
future.
The awareness that
pollution![]() ,
especially air and water pollution, does not respect national borders urges
governments to adopt internationally accepted environmental policies. The most
important environmental agencies, heavy industry and the world of chemistry in general, are developing and following a
code of conduct focused on precise strategies for pollution prevention.
,
especially air and water pollution, does not respect national borders urges
governments to adopt internationally accepted environmental policies. The most
important environmental agencies, heavy industry and the world of chemistry in general, are developing and following a
code of conduct focused on precise strategies for pollution prevention.
 |
Fig. 1:
Paul Anastas, one of the founders of Green Chemistry,
has served in the National Security
and International Activities Division in the White House Office of Science
and Technology Policy since 1999. In 1991, he established the
Green Chemistry Program, a partnership
between industry, government and university, which was expanded to include
the Presidential Green Chemistry Challenge
Awards. He is now Director of the
Green Chemistry Institute, Washington, DC. |
Green chemistry
essentially refers to the new sustainability
priorities in technological and scientific innovation, on the basis of general
rules stressing the need to abandon
harmful products![]() and processes. Some
strategies which can be adopted are:
and processes. Some
strategies which can be adopted are:
- Optimisation of balance of global mass in order to minimize waste.
- Minimisation of energy consumption, e.g. designing processes at ambient temperature and pressure.
- Use of raw materials taken from renewable sources.
- Whenever possible, replacement of old compounds with others which maintain their functional efficiency while minimizing their toxic impact on the environment and human health.
Environment-oriented molecular design has led to innovations such as:
|
|
|
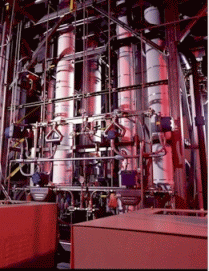
| Fig. 2:
A
supercritical CO2 reactor. Supercritical CO2 can
be used as a reaction solvent, as starting material to be incorporated
in a target molecule, for the extraction of natural products from
vegetal tissues (caffeine from coffee, etc), or for the purification of
active principles in drugs. (Credit: Organichem Corporation) |
- Replacement
of organic solvents with supercritical liquids. Supercritical carbon dioxide
(T ≥ 13.1°C, P ≥ 74 bar) is often an exceptional substitute
for organic solvents, in terms of
hazard and environmental impact reduction. It can be used in dry cleaning as
an alternative to chlorinated solvents, in semiconductor production and,
finally, as a reaction or extraction
solvent.
- Replacement
of brominated flame retardants. Flame retardants are used as plastic material
additives in a variety of products, furnishings, textiles and electronic
equipment. The most commonly used are aromatic compounds containing bromine
which, apart from remaining persistently in the environment, are capable of
bioaccumulating
 in organisms and having a harmful effect on our health. In
order to avoid these problems, the big chemical companies are racing to find
alternatives free of bromine, made up for example of a mix of epoxy resins and
inter metal oxides.
in organisms and having a harmful effect on our health. In
order to avoid these problems, the big chemical companies are racing to find
alternatives free of bromine, made up for example of a mix of epoxy resins and
inter metal oxides. - Replacement
of non-selective persistent pesticides. Polychlorinated
pesticides (aldrin) have been widely
used in agriculture for the last 50 years; they are very resistant to chemical
and microbiological breakdown in the environment and they are bioaccumulated
by organisms. Nowadays, a lot of effort is
directed to finding alternative
products, such as synthetic pyrethroids, analogues of the natural product
pyrethrine. Since natural pyrethrine is particularly instable in the
environment, analogues are designed which are more stable than the natural
compound and above all much less toxic for
higher organisms.

Fig. 3: A pesticide spray operation for fruit trees and fields; from an environmental point of view, a pesticide must be effective, selective (able to spare other forms of life including insects needed for pollination) and not persistent, in order to avoid bioaccumulation or transport to groundwaters.
(Credit: USDA, Photo by: Tim McCabe)The Webweavers: Last modified Tue, 20 Jul 2005 10:06:15 GMT